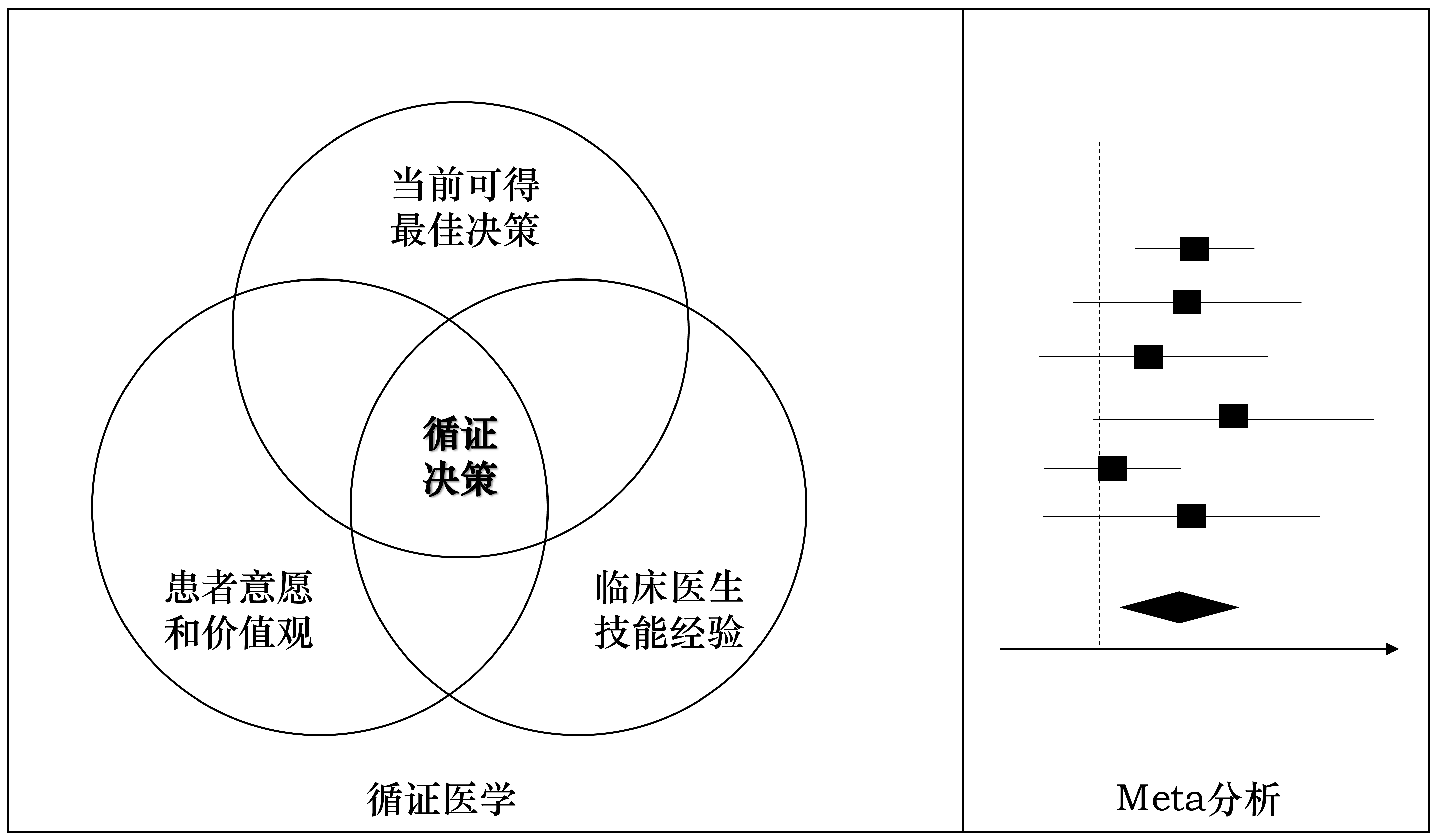
Evidence-Based Medicine (EBM)
Evidence-based medicine is the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients.
Sackett et al., 1996, p. 71
Meta-analysis represents a cornerstone methodology in evidence-based medicine. It is a sophisticated statistical approach that synthesizes findings from multiple independent studies to derive a quantitative summary of the collective evidence. The methodological framework encompasses: (1) systematic identification and retrieval of relevant studies; (2) rigorous quality assessment; (3) quantitative synthesis of data; (4) evidence-based interpretation of findings; and (5) comprehensive reporting following established guidelines.

